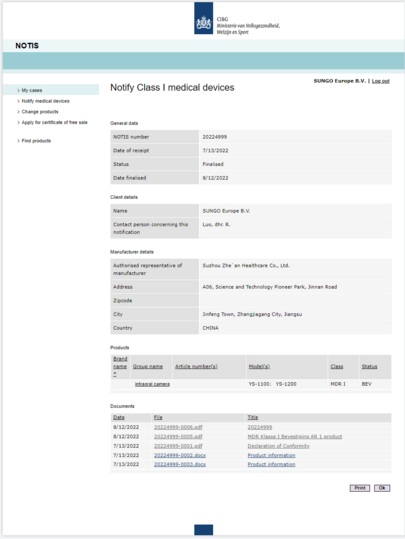
In order to enhance the safety and quality of our products for the international market, our diagnostic imaging products YS-1100 and YS-1200 have recently been awarded CE certification by the EU certification body. After obtaining ISO 13485:2016/EN ISO 13485:2016 medical device quality management system certification, our products have now been awarded CE certification from EU, which signifies the recognition of Zhe’an Healthcare in the EU market and means that we can provide more professional medical products of high quality for the international medical industry, further enhancing our international competitiveness.

CE certification credentials for the medical products of Zhe’an Healthcare
On the one hand, in order to respond to the national call of "Belt and Road Initiative", Zhe’an Healthcare has been actively exploring overseas markets. The passing of the EU CE certification not only helps us open up the European market, but also serves as the first step to open up the global market, which means that the company's products can be exported to various countries to achieve the free flow of goods in the global scope, which has a positive effect on the expansion of the company's overseas sales product categories and international business expansion. In the meantime, it also provides a safety and quality guarantee for the sale of products in the domestic market and enhances the brand influence of the products. This highly representative certificate has opened up a new era of development for the company.

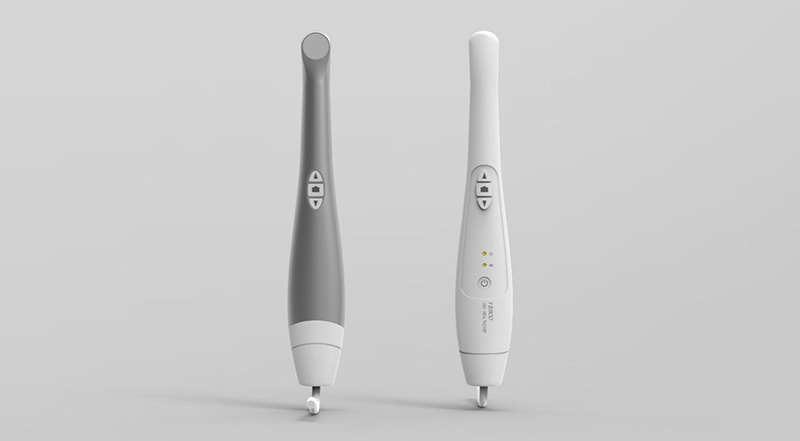
YS-1100
On the other hand, Zhe’an Healthcare has always been committed to the concept of green health. The company strictly implements and enforces the safety requirements and environmental standards in the R&D and manufacturing process, focusing on environmental safety while integrating environmental protection concepts into every aspect of our products, which coincides with the principles underlying CE. In addition, the CE certification is based on the strict inspection of products. This demonstrates that our company operates in line with domestic and international standards, while strictly controls the quality of exports, keeps pursuing higher standards and requirements, aiming at providing customers with better service and products of higher quality. As President Xi proposed , "Remain true to our original aspiration and keep our mission firmly in mind.", The staff of Zhe’an Healthcare take it as our original aspiration to provide customers with safe and effective products of stable quality, strive to achieve the goal of corporate development and social responsibility at the same time, and make greater contributions to China's medical and health care!


YS-1200
The award of the CE certificate from the EU not only proves our ability and determination, but also sets a higher goal and pursuit for the company, laying a solid foundation for the our development in the future.
About CE certification
The "CE" marking, a safety certification mark, is considered to be the manufacturer's passport to open and enter the European market. CE stands for CONFORMITE EUROPEENNE and indicates that the goods comply with the health, safety and environmental protection standards of the European Economic Area (EEA).
In the EU market, the "CE" marking serves as a compulsory certification mark. Whether it is for products produced in the EU or in other countries, in order to circulate freely in the EU market, the "CE" marking must be applied to show that the product meets the basic requirements of the EU Directive The New Approach to Technical Harmonization and Standardization. This is a mandatory requirement for products under EU law.
CE certification stands for the basic safety requirements limited to products that do not endanger the safety of humans, animals and goods. The harmonization directive stipulates only the main requirements and the general directive requirements are the task of the standard. Therefore, the precise meaning is that the CE marking is a mark for safety conformity instead of a mark for quality conformity. It is the "main requirement" that forms the core of the European Directive.
 Website QR Code
Website QR Code
All rights reserved:Suzhou Zhean Healthcare Co.,Ltd Technical Support:E-power